Current Study Systems
Malaria
Entomopathogenic fungi
Marek’s disease
Myxomatosis
Previous Study Systems
Daphnia
Gastrointestinal worms
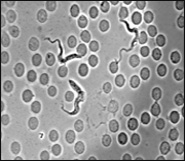
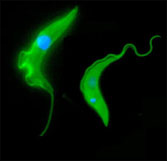
African Trypanosomes
Current Study Systems
Malaria
We work on malaria both because it is a hugely important disease of humans, and because malaria parasites are perhaps the group of eukaryotic microparasites about which are most known. Most importantly, cryopreserved isolates are available, and a large number of molecular genetic and immunological typing methods (and reagents) are available which we can use to in our experiments.
 We are interested in the ecology and evolution of virulence, transmission and in-host dynamics. Experimental analysis of these phenotypes requires an in vivo system. For the most part, we work with the rodent malaria Plasmodium chabaudi, which is an ideal experimental virulence model for any microparasite whose biology accords with classic models of virulence evolution (pathogen-encoded virulence determinants, virulence-transmission trade-offs).
We are interested in the ecology and evolution of virulence, transmission and in-host dynamics. Experimental analysis of these phenotypes requires an in vivo system. For the most part, we work with the rodent malaria Plasmodium chabaudi, which is an ideal experimental virulence model for any microparasite whose biology accords with classic models of virulence evolution (pathogen-encoded virulence determinants, virulence-transmission trade-offs).
P. chabaudi is certainly the best of the malaria models for investigating the questions we are interested in. It shares a number of relevant features with the most virulent human malaria, Plasmodium falciparum: (i) Blood forms of P. chabaudi do not have a marked preference for different cell types. (ii) Schizogony is highly synchronous. (iii) Sequestration of mature trophozoites/schizonts occurs. (iv) There is antigenic variation, and blood infections persist for several weeks. (v) Gametocyte production occurs mainly after the peak parasitaemia. (vi) In mosquitoes, low oocyst numbers are produced, but sporozoites have good infectivity to mice. Finally, and perhaps most importantly, recent analyses of malaria therapy data in humans have shown that, like P. chabaudi, a major determinant of severe disease and death is the inability to control the consequences of first peak of asexual parasites.
 Thicket rats are the natural hosts of P. chabaudi, but as with Drosophila in jars, selection on life history traits can fruitfully be examined in a novel environment. It is difficult to assess the extent to which the non-natural host might limit the ability to extrapolate the results to other malaria. However, one advantage is that there is probably more genetic variation revealed by novel environments, and so it may be possible to study virulence phenotypes that selection would have otherwise removed.
Thicket rats are the natural hosts of P. chabaudi, but as with Drosophila in jars, selection on life history traits can fruitfully be examined in a novel environment. It is difficult to assess the extent to which the non-natural host might limit the ability to extrapolate the results to other malaria. However, one advantage is that there is probably more genetic variation revealed by novel environments, and so it may be possible to study virulence phenotypes that selection would have otherwise removed.
For the most part, we use Anopheles stephensi because it is a conveniently maintained malaria vector. P. chabaudi does not require a secure insectary.
Plasmodium chabaudi: Life-histories and stabilates (deep-frozen samples) of isolates, lines and clones maintained at the University of Edinburgh, UK.
Entomopathogenic fungi
Malaria control has been successfully achieved with insecticides directed against blood-fed mosquitoes resting on house walls. However, the evolution of insecticide resistance in the mosquitoes and concerns about the environmental consequences of sustained use of chemicals make standard approaches unsustainable. We are trying to develop a solution to both these problems.
 Recent advances in production and formulation technology have increased the scope for use of Deuteromycete entomopathogenic fungi as oil-based biopesticides. These fungi are contact-trasnmitted, with resting spores that germinate on contact with insect cuticle. Using Anopheles stephensi and rodent malaria as a model system, and integrating empirical and theoretical approaches from fundamental ecology and agricultural pest control, we are investigating the potential of this new technology for development of a biopesticide for malaria control. We adopt a novel approach examining mixtures of fungal isolates and exploring effects of infection on vector capacity and not simply vector mortality. The ability of what might be quite avirulent pathogens to influence vector capacity has been virtually ignored, yet subtle effects of sub-lethal infection on, for example, feeding behaviour, flight potential, thermal behaviour and immune response are common in other host-pathogen systems and could have significant effects on vector capacity and malaria dynamics. This, coupled with a novel pathogen delivery system infecting resting mosquitoes via residual pick-up of spores from surfaces treated with the oil-based formulation, creates exciting new opportunities for sustainable malaria control.
Recent advances in production and formulation technology have increased the scope for use of Deuteromycete entomopathogenic fungi as oil-based biopesticides. These fungi are contact-trasnmitted, with resting spores that germinate on contact with insect cuticle. Using Anopheles stephensi and rodent malaria as a model system, and integrating empirical and theoretical approaches from fundamental ecology and agricultural pest control, we are investigating the potential of this new technology for development of a biopesticide for malaria control. We adopt a novel approach examining mixtures of fungal isolates and exploring effects of infection on vector capacity and not simply vector mortality. The ability of what might be quite avirulent pathogens to influence vector capacity has been virtually ignored, yet subtle effects of sub-lethal infection on, for example, feeding behaviour, flight potential, thermal behaviour and immune response are common in other host-pathogen systems and could have significant effects on vector capacity and malaria dynamics. This, coupled with a novel pathogen delivery system infecting resting mosquitoes via residual pick-up of spores from surfaces treated with the oil-based formulation, creates exciting new opportunities for sustainable malaria control.
Photo courtesy of Edinburgh Research and Innovation Ltd.
Wellcome Trust Highlight article 2006/7 click here.
Marek’s disease
Marek’s disease is a ubiquitous endemic pathogen of poultry first identified by Josef Marek in 1907. The causative agent, Marek’s Disease Virus (MDV), is an alpha-herpesvirus that is highly collinear with Herpes Simplex Virus I. Like most herpesviruses, the early stages of infection with MDV are characterised by a semi-productive cytolytic infection in immune organs followed by a period of latency. Serotype 1 MDVs are oncogenic, and infection of susceptible birds with serotype 1 MDVs eventually leads to reactivation of the virus and the dissemination of infected and transformed CD4+ T-cells to visceral organs and nervous tissue.
 Marek’s disease was the first herpesvirus and also the first oncogenic disease against which a successful vaccination strategy was achieved. Vaccination in commercial settings occurs in ovo or at one day post-hatch using live attenuated or non-pathogenic strains. These vaccines are classic examples of “leaky” vaccines, preventing morbidity and mortality in vaccinated birds but not preventing the replication or transmission of the virus.
Marek’s disease was the first herpesvirus and also the first oncogenic disease against which a successful vaccination strategy was achieved. Vaccination in commercial settings occurs in ovo or at one day post-hatch using live attenuated or non-pathogenic strains. These vaccines are classic examples of “leaky” vaccines, preventing morbidity and mortality in vaccinated birds but not preventing the replication or transmission of the virus.
Since the original description of MDV, it has been shown unequivocally that more virulent pathotypes of the virus have emerged, particularly where chickens are farmed intensively. Current theory is that the use of leaky vaccines has driven the evolution of these virulent pathotypes.
This system therefore provides a useful model to study vaccine-driven evolution of pathogens and to build on the work already carried out in malaria. The system has several advantages including the ability to work in the natural host animal and the fact that evolution of the pathogen is known to occur in the field.
Myxomatosis
Myxoma virus is a double-strand DNA virus (family Poxviridae) of rabbits. In the natural host, the South American rabbit (Sylvilagus brasiliensis), myxoma viruses induce an innocuous localized cutaneous fibroma at the site of inoculation. However, in European rabbits (Oryctolagus cuniculus), the virus replicates at the inoculation site and disseminates to cause the lethal disease myxomatosis. Myxoma virus is passively transmitted when mosquitoes or fleas probing through the virus-rich lesions for a blood meal.
Viral strains which effectively killed all infected rabbits were used to initiate the epidemics in both Australia (1950) and Europe (1952). With outstanding foresight, Frank Fenner and colleagues tracked the phenotypic evolution of virulence in the Australian epidemic by testing the lethality of isolates from the field over the subsequent decades in laboratory rabbits. This work showed that the initial viral strains that killed essentially 100% of rabbits in less than a fortnight were rapidly replaced with strains of lower virulence (case fatality rates of 70-95%), with some strains killing less than half of rabbits. In subsequent decades lethality rose again, with all strains having case fatality rates >50%.
Myxomatosis a canonical case for evolutionary science and public health risk evaluation. (1) A highly lethal pathogen, one that effectively killed all hosts, caused two continent-wide epidemics which continue to this day. (2) Virus adaptation to the new host was associated with rapid and profound virulence evolution. (3) Even the ‘mildest’ strains that evolved kill about 50% of naive hosts.
In other words, a lethal fulminant disease was somewhat tamed by natural selection, but at a level still frightening for a human disease.
Previous Study Systems
Daphnia
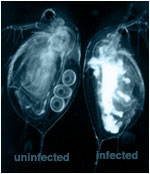 Daphnia magna, and its pathogenic bacterial microparasite Pasteuria ramosa combine a number of features which makes them an ideal for laboratory and field studies of host-parasite ecology and evolution.
Daphnia magna, and its pathogenic bacterial microparasite Pasteuria ramosa combine a number of features which makes them an ideal for laboratory and field studies of host-parasite ecology and evolution.
First, Daphnia are rather small (1-2mm) and have a generation time of about one week, which results in 15 to 20 generations per growing season, so that the evolution of populations can be observed within a reasonable timeframe. The small size and ease of handling of Daphnia also permits the collection of large samples from the wild, and the maintenance of thousands of individuals in the lab.
Second, because Daphnia have a clear carapace, the parasite spore mass in the haemolymph or ovaries of infected individuals is highly visible, and thus the infection status of hosts in field samples, as well as those in experiments, can be classified with the naked eye. This aspect greatly expedites the processing of field samples and the scoring of individuals during experiments.
Third, females infected in field collections can be treated with antibiotics, after which they resume reproduction. This makes it possible to perform experiments that include host genotypes derived from naturally-infected subset of populations (this would normally not be possible because infected hosts do not reproduce). Using this technique, it has been possible to show that susceptibility in the field correlates strongly with susceptibility to laboratory infection.
Fourth, reproduction in Daphnia can be controlled so that it is exclusively clonal, where females produce daughters that are genetically identical to their mother, and thus it is possible to truly replicate a host population sample by splitting the offspring of collected individuals into separate replicates.
Fifth, clonal reproduction of hosts and the ability to freeze P. ramosa transmission spores are especially valuable aspects of this system. The genetic structure of both host and parasite populations collected from the field can be ‘frozen’ until they are used in an experiment. Sixth, field studies using allozymes on Daphnia populations have shown that clonal frequencies may fluctuate wildly. The cause of these fluctuations has not been established. Finally, there is now a Daphnia genome project, and there is huge potential to exploit the Drosophila and Anopheles genome projects.
Importantly, and in contrast to the situation with Drosophila, a substantial amount is known about the natural parasites (and field ecology) of Daphnia. More is known about Anopheles parasites, but we know of no directly transmitted parasites of Anopheles which do not represent a major biosecurity risk to other users of our Anopheles insectaries.
For other reasons why Daphnia is a good model, and for further info in the Daphnia genome project, click here.
For further info on Daphnia parasites, click here.
Gastrointestinal worms
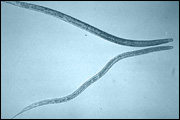 Gastrointestinal worms are hugely important, much studied macroparasites which are relatively easy to work with. The species we use have long been studied in the laboratory as model GI nematodes and it is relatively easy to obtain fresh parasite isolates from the field. Rats and mice are their natural host. We use a range of GI nematodes because the contrasting natural histories capture different features of the range of GI nematodes which cause problems for humans and domestic animals. In vivo model systems we use include: Strongyloides ratti, S.venezulensis, Heligomosomoides polygyrus, and Nippostrongylus brasiliensis.
Gastrointestinal worms are hugely important, much studied macroparasites which are relatively easy to work with. The species we use have long been studied in the laboratory as model GI nematodes and it is relatively easy to obtain fresh parasite isolates from the field. Rats and mice are their natural host. We use a range of GI nematodes because the contrasting natural histories capture different features of the range of GI nematodes which cause problems for humans and domestic animals. In vivo model systems we use include: Strongyloides ratti, S.venezulensis, Heligomosomoides polygyrus, and Nippostrongylus brasiliensis.
Trypanosomes
Trypanosomiasis is a major socio-economic disease of humans and animals in sub-Saharan Africa. Our aim was to study host-parasite interactions using in vivo and in vitro models focussing primarily on within-host competition in collaboration with Keith Matthews and Dave Barry.